Greener energy for the future
METAL-BASED BATTERIES
Research overview
Aluminum is the most abundant metal in the Earth’s crust. Comparing with aluminum, only lithium has a slightly higher electrochemical equivalent (3.86 Ahg⁻¹). Furthermore, the theoretical specific volumetric capacity of aluminum is the highest among metallic fuels (8.04 Ahcm³). These advantages along with its low price-per–energy unit have increased the interest in using it as an anode material in metal-air battery systems, specifically, in alkaline metal-air batteries
Practical designs and high-performance electrode materials are considering to develop aluminum-air batteries. Gelled electrolytes based on NaCl and water-absorbing polymers have been used in aluminum-air batteries for safety and portability. In addition, several methods of modifying aluminum anodes to improve anode performance have been proposed. For example, an etching method was applied to an aluminum anode to improve the surface area, and zinc was doped on the aluminum surface to improve the stability and performance of the anode.
Practical designs and high-performance electrode materials are considering to develop aluminum-air batteries. Gelled electrolytes based on NaCl and water-absorbing polymers have been used in aluminum-air batteries for safety and portability. In addition, several methods of modifying aluminum anodes to improve anode performance have been proposed. For example, an etching method was applied to an aluminum anode to improve the surface area, and zinc was doped on the aluminum surface to improve the stability and performance of the anode.
Current research topics
1. Developing quasi-solid aluminum-air batteries
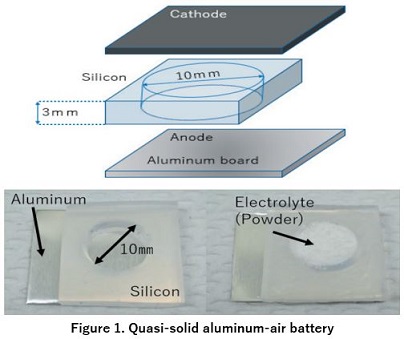
(“Simplification of aluminum air battery by gelation electrolyte and investigation of material for cathode electrode,” T Okobira, DT Nguyen, and T Kozo, Journal of the Japan Society of Applied Electromagnetics and Mechanics, JSAEM, 27(1), 108–114, 2019. )
2. Modifying the aluminum anode electrode by etching and doping methods
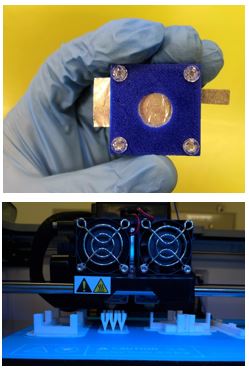
Figure 2. Fabrication of metal-based batteries.

(“Simplification of aluminum air battery by gelation electrolyte and investigation of material for cathode electrode,” T Okobira, DT Nguyen, and T Kozo, Journal of the Japan Society of Applied Electromagnetics and Mechanics, JSAEM, 27(1), 108–114, 2019. )
2. Modifying the aluminum anode electrode by etching and doping methods

Figure 2. Fabrication of metal-based batteries.
