Selected Papers
Selected Papers & Top 70 Papers
Selected Papers
- Kita, Yasuyuki; Tohma, Hirofumi; Inagaki, Masanao; Hatanaka, Kenji; Yakura, Takayuki
Total synthesis of Discorhabdin C: a General Aza Spiro Dienone Formation from O-Silylated Phenol Derivatives Using a Hypervalent Iodine Reagent.
J. Am. Chem. Soc., 114, 2175-80 (1992).
Hypervalent iodine oxidation of 0-silylated phenols bearing various types of aminoquinones at the para position in 2,2,2-trifluoroethanoI gave azacarbocyclic spiro dienones in good yields. Using this method, the first total synthesis of discorhabdin C, which was isolated from the sponge of Latrunculia du Bocage in New Zealand, was achieved.
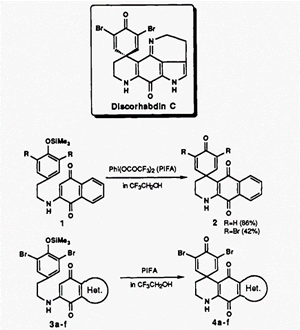
- Kita, Yasuyuki; Tohma, Hirofumi; Hatanaka, Kenji; Takada, Takeshi; Fujita, Shigekazu; Mitoh, Shizue; Sakurai, Hiromu; Oka, Shigenori
Hypervalent Iodine-Induced Nucleophilic Substitution of para-Substituted Phenol Ethers. Generation of Cation Radicals as Reactive Intermediates.
J. Am. Chem. Soc., 116, 3684-3691 (1994).
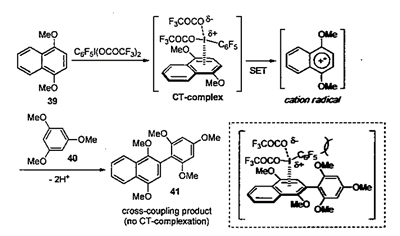
A novel hypervalent iodine induced nucleophilic substitution ofpara-substituted phenol ethers in the presence of a variety of nucleophiles is described. UV and ESR spectroscopic studies indicate that this reaction proceeds via cation radicals, [ArH*+],as reactive intermediates generated by single-electron transfer (SET) from a charge-transfer (CT) complex of phenol ethers with phenyliodine(II1) bis(trifluor0acetate) (PIFA). This is the first case that involves a radical intermediate on hypervalent iodine oxidations of aromatic compounds.
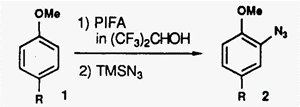
- Kita, Yasuyuki; Shibata, Norio; Kawano, Noriyuki; Tohjo, Takashi; Fujimori, Chino; Ohishi, Hirofumi
Pummerer-type Cyclization of Arnstein Tripeptide Analogs Induced by O-Silylated Ketene Acetals: Studies of Penicillin Biosynthesis.
J. Am. Chem. Soc., 116, 5116-5121 (1994).
We report the first biomimetic conversion of Arnstein tripeptide analogues (l a and l b) into cis β-lactams (2a and 2b)using O-silylated ketene acetal (3)involving asymmetric induction from the sulfoxide sulfur to the α-carbon. The peptide 1 was treated with 3 at room temperature in the presence of a catalytic amount of ZnI2 in MeCN to give cis-2, trans-2, and α-siloxysulfide (7). Reaction of R-1 with 3 gave cis-2 predominantly, and S-1 gave a mixture of cis-2and trans-2. High cis selectivity was obtained by the use of a large volume of solvent and was strongly influenced by the absolute stereochemistry of the sulfoxide, the cysteinyl amino group, and the volume of solvent. The cis β-lactams (2a,b)were obtained preferentially from R-la,b. These chemical transformations strongly support Baldwin's mechanism which involves the initial formation of the cis β-lactam by the Pummerer-type cyclization of the Arnstein tripeptide in penicillin biosynthesis and provide useful information on the first key step in penicillin biosynthesis.
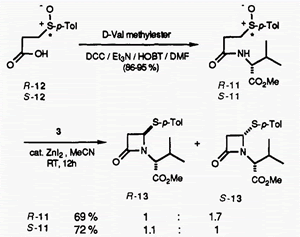
- Kita, Yasuyuki; Takeda, Yoshifumi; Matsugi, Masato; Iio, Kiyosei; Gotanda, Kentoku; Murata, Kenji; Akai, Shuji
Isolation of the Quinone Mono O,S-Acetal Intermediates of the Aromatic Pummerer-Type Rearrangement of p-Sulfinylphenols with 1-Ethoxyvinyl Esters.
Angew. Chem. Int. Ed., 36, 1529-1531 (1997).
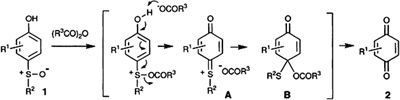
- Fujioka, Hiromichi; Nagatomi, Yasushi; Kitagawa, Hidetoshi; Kita, Yasuyuki
Highly Enantioselective Asymmetrization of meso-1,2-Diols through a Novel and Efficient Reaction Cycle.
J. Am. Chem. Soc., 119, 12016-12017 (1997).
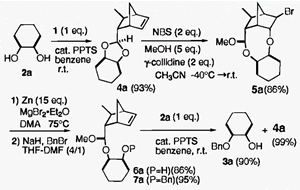
- Kita, Yasuyuki; Higuchi, Kazuhiro; Yoshida, Yutaka; Iio, Kiyosei; Kitagaki, Shinji; Akai, Shuji; Fujioka, Hiromichi
Asymmetric Total Synthesis of Fredericamycin A.
Angew. Chem. Int. Ed., 38, 683-686 (1999).
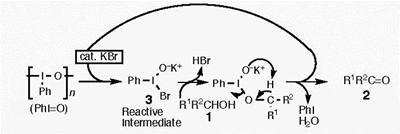
- Tohma, Hirofumi; Takizawa, Shinobu; Maegawa, Tomohiro; Kita, Yasuyuki
Facile and Clean Oxidation of Alcohols in Water Using Hypervalent Iodine(III) Reagents.
Angew. Chem. Int. Ed., 39, 1306-1308 (2000).
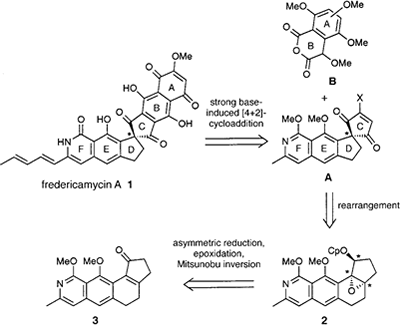
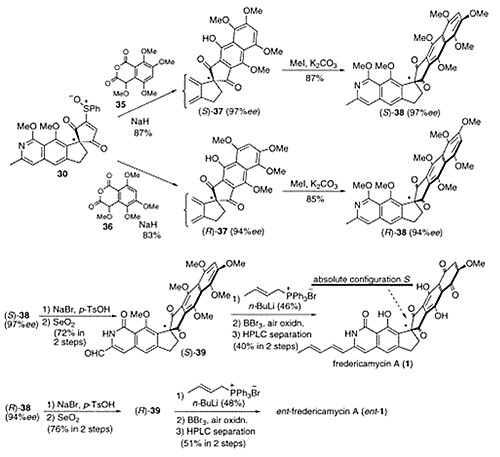
- Kita, Yasuyuki; Higuchi, Kazuhiro; Yoshida, Yutaka; Iio, Kiyosei; Kitagaki, Shinji; Ueda, Koichiro; Akai, Shuji; Fujioka, Hiromichi
Enantioselective Total Synthesis of a Potent Antitumor Antibiotic, Fredericamycin A.
J. Am. Chem. Soc., 123, 3214-3222 (2001).
The asymmetric total synthesis of both enantiomers of the potent antitumor antibiotic fredericamycin A (1) is detailed based on the protocol for the construction of its peri-hydroxy polyaromatic skeleton bearing the chirality at the spiro carbon via a strong base-induced cycloaddition of suitably substituted homophthalic anhydrides (AB-ring unit) with an optically active CDEF-ring unit. Particular attention has been given to the novel synthesis of the optically active spiro carbon center by a stereospecific rearrangement of optically active benzofuzed-trans-epoxy acylates leading to spirocyclopentane-1,1‘-indane systems. This method is quite useful for the construction of an optically active spiro compound and was applied to the synthesis of the optically pure CDEF-ring unit of 1. Cycloaddition of the optically pure CDEF-ring unit to AB-ring units prepared via benzyne afforded two natural and unnatural-type hexacyclic compounds, which were converted to natural and unnatural enantiomers of synthetic 1, and the absolute configuration of natural 1 was determined as S.
- Tohma, Hirofumi; Harayama, Yuu; Hashizume, Miki; Iwata, Minako; Egi, Masahiro; Kita, Yasuyuki
Synthetic Studies on the Sulfur-Cross-Linked Core of Antitumor Marine Alkaloid, Discorhabdins.
Angew. Chem. Int. Ed., 41, 348-350 (2002).
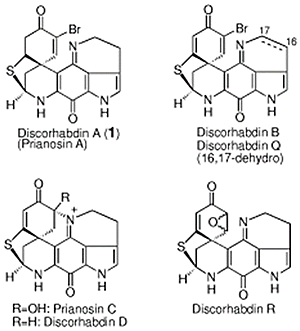
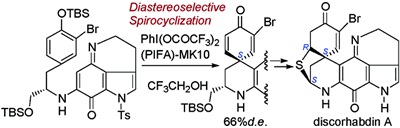
- Tohma, Hirofumi; Harayama, Yu; Hashizume, Miki; Iwata, Minako; Kiyono, Yorito; Egi, Masahiro; Kita, Yasuyuki
The First Total Synthesis of Discorhabdin A.
J. Am. Chem. Soc., 125, 11235-11240 (2003).
- Akai, Shuji; Tanimoto, Kouichi; Kita, Yasuyuki
Lipase-Catalyzed Domino Dynamic Kinetic Resolution of Racemic 3-Vinylcyclohex-2-en-1-ols/Intramolecular Diels-Alder Reaction: One-Pot Synthesis of Optically Active Polysubstituted Decalins.
Angew. Chem. Int. Ed., 43, 1407-1410 (2004).
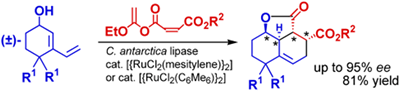
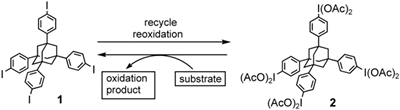
- Tohma, Hirofumi; Maruyama, Akinobu; Maeda, Akihisa; Maegawa, Tomohiro; Dohi, Toshifumi; Shiro, Motoo; Morita, Tetsuichiro; Kita, Yasuyuki
Preparation and Reactivity of 1,3,5,7-Tetrakis[4-(Diacetoxyiodo)Phenyl]Adamantane, a Recyclable Hypervalent Iodine(III) Reagent.
Angew. Chem. Int. Ed., 43, 3595-3598 (2004).
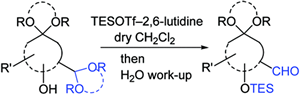
- Fujioka, Hiromichi; Sawama, Yoshinari; Murata, Nobutaka; Okitsu, Takashi; Kubo, Ozora; Matsuda, Satoshi; Kita, Yasuyuki
Unexpected Highly Chemoselective Deprotection of the Acetals from Aldehydes and not Ketones: TESOTf-2,6-Lutidine Combination.
J. Am. Chem. Soc., 126, 11800-11801 (2004).
- Kita, Yasuyuki; Akai, Shuji
1-Alkoxyvinyl Esters: Renaissance of Half-Century-Old Acyl Donors with Potential Applicability.
Chem. Rec., 4, 363-372 (2004).
Among the various kinds of acyl donors, the 1-alkoxyvinyl esters have characteristic features, such as a high reactivity under nearly neutral conditions and the generation of neutral and volatile esters as single coproducts. Although their use in organic syntheses began in the middle of the 1950s, no significant progress has been seen. This is probably because the existing method of preparing alkoxyvinyl esters used toxic mercuric salts and was not totally applicable for those esters having functionalized acyl moieties.
We have discovered that the use of a catalytic amount of the less toxic [RuCl2(p-cymene)]2 effectively accelerates the addition of carboxylic acids to ethoxyacetylene to give ethoxyvinyl esters bearing a variety of functionalized acyl groups in high yields. This discovery has opened a new avenue for developing new reactions and new synthetic methodologies based on the design and use of these acyl donors with suitable functional groups. Such examples include (i) the installation of hydrophilic acyl moieties on biologically active compounds, (ii) asymmetric Pummerer-type reactions, (iii) aromatic Pummerer-type reactions, (iv) the lipase-catalyzed desymmetrization of symmetrical 1,3-diols, and (v) lipase-catalyzed domino reactions. Future possibilities for these acyl donors are also discussed. © 2005 The Japan Chemical Journal Forum and Wiley Periodicals, Inc. Chem Rec 4: 363–372; 2004: Published online in Wiley InterScience (www.interscience.wiley.com) DOI 10.1002/tcr.20027
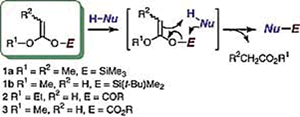
- Fujioka, Hiromichi; Ohba, Yusuke; Hirose, Hideki; Murai, Kenichi; Kita, Yasuyuki
A Double Iodoetherification of s-Symmetric Diene Acetals for Installing Four Stereogenic Centers in a Single Operation: Short Asymmetric Total Synthesis of Rubrenolide.
Angew. Chem. Int. Ed., 44, 734-737 (2005).
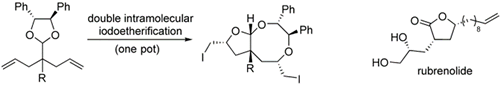
- Kita, Yasuyuki; Matsuda, Satoshi; Fujii, Eri; Horai, Mai; Hata, Kayoko; Fujioka, Hiromichi
Domino Reaction of 2,3-Epoxy-1-Alcohols and PIFA in the Presence of H2O and the Concise Synthesis of (+)-Tanikolide.
Angew. Chem. Int. Ed., 44, 5857-5860 (2005).
- Dohi, Toshifumi; Maruyama, Akinobu; Yoshimura, Misaki; Morimoto, Koji; Tohma, Hirofumi; Kita, Yasuyuki
Versatile Hypervalent-Iodine(III)-Catalyzed Oxidations with m-Chloroperbenzoic Acid as a Cooxidant.
Angew. Chem. Int. Ed., 44, 6193-6196 (2005).
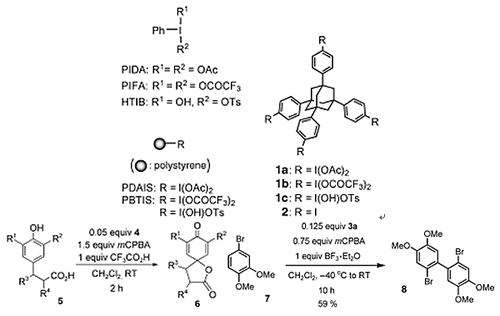
- Akai, Shuji; Tanimoto, Kouichi; Kanao, Yukiko; Egi, Masahiro; Yamamoto, Tomoko; Kita, Yasuyuki
A Dynamic Kinetic Resolution of Allyl Alcohols by the Combined Use of Lipases
and [VO(OSiPh3)3].
Angew. Chem. Int. Ed., 45, 2592-2595 (2006).
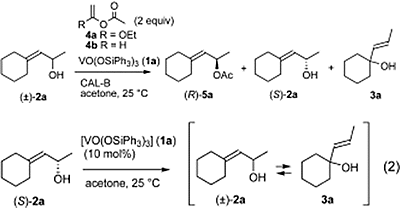
- Fujioka, Hiromichi; Okitsu, Takashi; Sawama, Yoshinari; Murata, Nobutaka; Li, Ruichuan; Kita, Yasuyuki
Reaction of the Acetals with TESOTf-Base Combination; Speculation of the Intermediates and Efficient Mixed Acetal Formation.
J. Am. Chem. Soc., 128, 5930-5938 (2006).
- Akai, Shuji; Kakiguchi, Keisuke; Nakamura, Yuka; Kuriwaki, Ikumi; Dohi, Toshifumi; Harada, Shusaku; Kubo, Ozora; Morita, Nobuyoshi; Kita, Yasuyuki
Total Synthesis of (+/-)-γ-Rubromycin on the Basis of Two Aromatic Pummerer-Type Reactions.
Angew. Chem. Int. Ed., 46, 7458-7461 (2007).
- Dohi, Toshifumi; Ito, Motoki; Morimoto, Koji; Iwata, Minako; Kita, Yasuyuki
Oxidative Cross-Coupling of Arenes Induced by Single-Electron Transfer Leading to Biaryls Using Organoiodine(III) Oxidants.
Angew. Chem. Int. Ed., 47, 1301-1304 (2008).
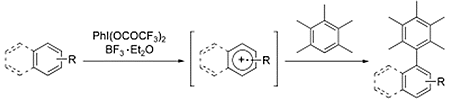
- Dohi, Toshifumi; Maruyama, Akinobu; Takenaga, Naoko; Senami, Kento; Minamitsuji, Yutaka; Fujioka, Hiromichi;Caemmerer, Simon B; Kita, Yasuyuki
A Chiral Hypervalent Iodine(III) Reagent for Enantioselective Dearomatization of Phenols.
Angew. Chem. Int. Ed., 47, 3787-3790 (2008).
- Akai, Shuji; Ikawa, Takashi; Takayanagi, Sho-ichi; Morikawa, Yuki; Mohri, Shinya; Tsubakiyama, Masaya; Egi,Masahiro; Wada, Yasufumi; Kita, Yasuyuki
Synthesis of Biaryl Compounds Through Three-Component Assembly: Ambidentate Effect of the Tert-butyldimethylsilyl Group for Regioselective Diels-Alder and Hiyama Coupling Reactions.
Angew. Chem. Int. Ed., 47, 7673-7676 (2008).
- Dohi, Toshifumi; Kita, Yasuyuki
Hypervalent Iodine Reagents as a New Entrance to Organocatalysts.
Chem. Commun., 16, 2073-2085 (2009).
The catalytic utilization of hypervalent iodine reagents, largely in consideration of economical and environmental viewpoints, is a most attractive strategy due to their unique features as extremely useful oxidants, with mild, safe, and environmentally friendly characteristics. In addition to a system based on electrochemical reoxidation conditions, new reliable catalytic methods have emerged in recent years that can broaden the scope of the catalytic concept. For these contributions, a catalytic strategy is now available for performing many representative types of oxidative bond-forming reactions and alcohol oxidations mediated by hypervalent iodines, some of which even include key transformations for natural product synthesis. A suitable choice of terminal oxidants, e.g., m-chloroperbenzoic acid (mCPBA) or Oxone®, for generation of active hypervalent iodine(III) or (V) species from iodoarenesin situ, has led to recent rapid expansion in this field. This feature article highlights the historical background and the efforts made to realize the catalytic utilization of these reagents, especially with focus on iodine(III).
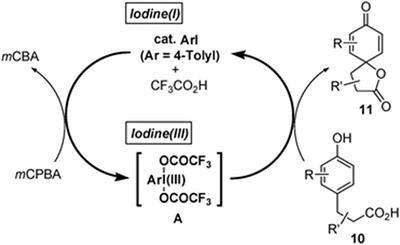
- Kita, Yasuyuki; Morimoto, Koji; Ito, Motoki; Ogawa, Chieko; Goto, Akihiro; Dohi, Toshifumi
Metal-Free Oxidative Cross-Coupling of Unfunctionalized Aromatic Compounds.
J. Am. Chem. Soc., 131, 1668-1669 (2009).
The oxidative cross-coupling reaction between two unfunctionalized arenes [oxidative C−H/C−H cross-coupling] has been widely accepted as a new and environmentally benign method for the direct synthesis of extremely important biaryls involving heteroaryls as electronic materials and devices (eq 1), by which the conventional stepwise approaches through prefunctionalizations of arenes, such as halides or metals, would be partially replaced.(1) On the contrary, the number of the selective cross-coupling methods is yet limited due to the difficulty of suppressing the undesired homodimer formations. The successful methods include σ-aryl palladiums,(1, 2) phenoxy copper species,(3) anilenium ions,(4) and aromatic cation radicals(5) as the key intermediates.
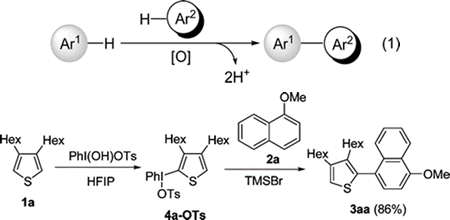
- Dohi, Toshifumi; Yamaoka, Nobutaka; Kita, Yasuyuki
Fluoroalcohols: Versatile Solvents in Hypervalent Iodine Chemistry and Syntheses of Diaryliodonium(III) Salts.
Tetrahedron, 66, 5775-5785 (2010).
We first introduced 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) and 2,2,2-trifluoroethanol (TFE) as unique solvents for reactions involving hypervalent iodine-mediated phenolic oxidations in the 1980s, in which the fluoroalcohols have been successfully utilized as stabilizing solvents of the reactive cationic intermediates, generated in situ by the action of phenyliodine(III) diacetate (PIDA) and phenyliodine bis(trifluoroacetate) (PIFA). This pioneering study produced a breakthrough in hypervalent iodine chemistry, and many synthetic applications that enable a variety of transformations have appeared utilizing this unique medium. For example, the single-electron-transfer (SET) oxidation ability of PIFA toward phenyl ethers has been discovered for the first time in HFIP and TFE, taking advantage of the unique acid-like behaviors to stabilize the aromatic cation radicals. More recently, the catalytic strategy of hypervalent iodine reagents has found extensive applications with the aid of the fluoroalcohols to produce highly reactive catalytic species under mild conditions. The fluoroalcohols are now widely used as versatile solvents not only in hypervalent iodine chemistry, but also in other organic syntheses. This manuscript for the Special Issue deals with the background of the hypervalent iodine chemistry and a new topic concerning the utility of fluoroalcohols for the synthesis of diaryliodonium(III) salts.
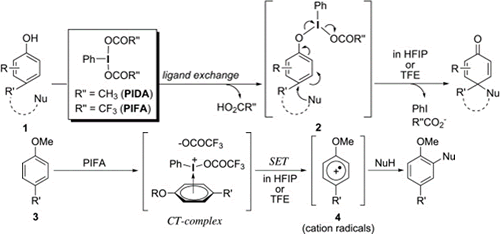
- Dohi, Toshifumi; Ito, Motoki; Yamaoka, Nobutaka; Morimoto, Koji; Fujioka, Hiromichi; Kita, Yasuyuki
Unusual ipso Substitution of Diaryliodonium Bromides Initiated by a Single-Electron-Transfer Oxidizing Process.
Angew. Chem. Int. Ed., 49, 3334-3337 (2010).
Aromatic substitution: The treatment of diaryliodonium bromides 1 with aromatic nucleophiles 2, afforded a variety of heteroaryl-containing biaryls 3 in good yields. The ipso-substitution process at the heteroaryl ring in 1 occurs through the formation of aromatic cation radicals, which are initiated by the single-electron-transfer (SET) oxidation of 2. (HFIP= hexafluoroisopropanol)
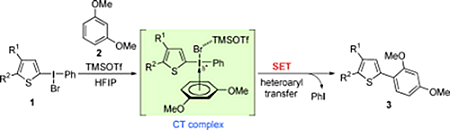
- Ikawa, Takashi; Takagi, Akira; Kurita, Yurio; Saito, Kozumo; Azechi, Kenji; Egi, Masahiro; Kakiguchi, Keisuke; Kita, Yasuyuki; Akai, Shuji
Preparation and Regioselective Diels-Alder Reactions of Borylbenzynes: Synthesis of Functionalized Arylboronates.
Angew. Chem. Int. Ed., 49, 5563-5566 (2010).
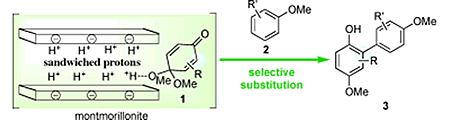
- Dohi, Toshifumi; Washimi, Naohiko; Kamitanaka, Tohru; Fukushima, Kei-ichiro; Kita, Yasuyuki
Coupling of Quinone Monoacetals Promoted by Sandwiched Bronsted Acids: Synthesis of Oxygenated Biaryls.
Angew. Chem. Int. Ed., 50, 6142-6146 (2011).
Unusual protons: Brønsted acids sandwiched between sheets of solid acids, such as montmorillonites, activated quinone monoacetals 1 to selectively react with aromatic nucleophiles 2 in an unprecedented substitution reaction. The synthetic utility of the strategy for obtaining highly oxygenated biaryls 3 is highlighted by the synthesis of gilvocarcin aglycones.
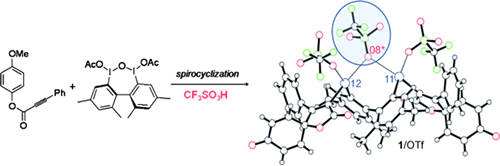
- Dohi, Toshifumi; Kato, Daishi; Hyodo, Ryo; Yamashita, Daisuke; Shiro, Motoo; Kita, Yasuyuki
Discovery of Stabilized Bisiodonium Salts as Intermediates in the Carbon-Carbon Bond Formation of Alkynes.
Angew. Chem. Int. Ed., 50, 3784-3787 (2011).
Ways and means to spirocycles: Pseudocyclic bisiodonium salts 1 stabilized by a secondary bonding interaction—an IIII⋅⋅⋅O⋅⋅⋅IIII pseudobridge linkage (circle) —were prepared by C—C bond formation between aryl alkynes and a μ-oxo-bridged hypervalent iodine compound. The synthetic utility of salts 1 was demonstrated by their facile conversion into functionalized spirocycles by treatment with a nucleophile.
- Ikawa, Takashi; Nishiyama, Tsuyoshi; Shigeta, Takashi; Mohri, Shinya; Morita, Shinsuke; Takayanagi, Sho-ichi; Terauchi, Yuki; Morikawa, Yuki; Takagi, Akira; Ishikawa, Yoshinobu; Fujii, Satoshi; Kita, Yasuyuki; Akai, Shuji
Ortho-Selective Nucleophilic Addition of Primary Amines to Silylbenzynes: Synthesis of 2-Silylanilines.
Angew. Chem. Int. Ed., 50, 5674-5677 (2011).
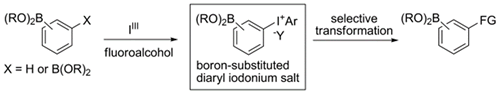
- Ito, Motoki; Itani, Itsuki; Toyoda, Yosuke; Morimoto, Koji; Dohi, Toshifumi; Kita, Yasuyuki
Synthesis of Boron-Substituted Diaryliodonium Salts and Selective Transformation into Functionalized Aryl Boronates.
Angew. Chem. Int. Ed., 51, 12555-12558 (2012).
- Dohi, Toshifumi; Fukushima, Kei-ichiro; Kamitanaka, Tohru; Morimoto, Koji; Takenaga, Naoko; Kita, Yasuyuki
An Excellent Dual Recycling Strategy for the Hypervalent Iodine/Nitroxyl Radical
Mediated Selective Oxidation of Alcohols to Aldehydeds and Ketones.
Green Chem, 14, 1493-1501 (2012).
Using a recyclable hypervalent iodine reagent 1, the authors have constructed versatile and green methods for the hypervalent iodine and nitroxyl radical-mediated selective oxidation of alcohols to aldehydes and ketones. The recyclable reagent 1 having a unique tetraphenyladamantane structure exhibited almost the same reactivity as the ordinary reagent, phenyliodine diacetate (PIDA), in the hypervalent iodine(III)/2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO)-mediated oxidation. For recycling, the reagent 1 could be nearly quantitatively recovered as the reduced tetraiodide 2 by filtration after reaction completion, utilizing the insolubility of the formed 2 in a polar solvent, specifically, methanol. Based on the confirmed reactivity and excellent recycling operation of the reagent 1, the methodology has been further extended to a greener dual recycling strategy. By combining the recyclable iodine reagent 1 and silica-supported TEPMO catalyst, a variety of alcohols were effectively oxidized to the desired aldehydes, and the two types of used reagent and catalyst, the iodine 1 and immobilized TEMPO 5, could be separately recovered by an easy workup, and both repeatedly used without any loss of their original activities for at least four cycles.
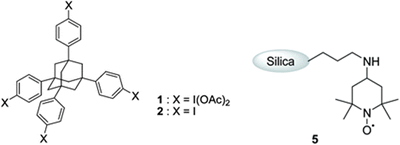
- Sawama, Yoshinari; Shishido, Yuko; Yanase, Takayoshi; Kawamoto, Koichi; Goto, Ryota; Monguchi, Yasunari; Kita, Yasuyuki; Sajiki, Hironao
Efficient Generation of ortho-Naphthoquinone Methides from 1,4-Epoxy-1,4- Dihydronaphthalenes and their Annulation with Allyl Silanes.
Angew. Chem. Int. Ed., 52, 1515-1519 (2013).
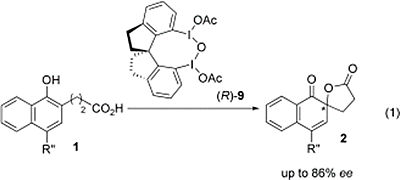
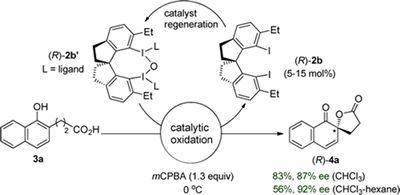
- Dohi, Toshifumi; Takenaga, Naoko; Nakae, Tomofumi; Toyoda, Yosuke; Yamasaki, Mikio; Shiro, Motoo; Fujioka, Hiromichi; Maruyama, Akinobu; Kita, Yasuyuki
Asymmetric Dearomatizing Spirolactonizaion of Naphthols Catalyzed by Spirobiindane-Based Chiral Hypervalent Iodine Species.
J. Am. Chem. Soc., 135, 4558-4566 (2013).
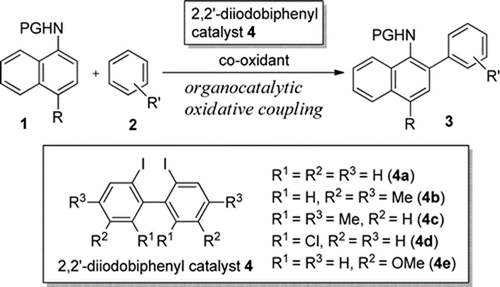
- Ito, Motoki; Kubo, Hiroko; Itani, Itsuki; Morimoto, Koji; Dohi, Toshifumi; Kita, Yasuyuki
Organocatalytic C-H/C-H’ Cross-Biaryl Coupling: C-Selective Arylation of Sulfonanilides with Aromatic Hydrocarbons.
J. Am. Chem. Soc., 135, 38, 14078-14081 (2013).
The hypervalent iodine-mediated C–C selective coupling of N-methanesulfonyl anilides with aromatic hydrocarbons has been developed. The first organocatalytic oxidative cross-biaryl-coupling was achieved by the catalyst control in defining specific 2,2′-diiodobiphenyls for the direct C–C bond formations.
- Suzuki, Satoru; Kamo, Tomohiro; Fukushi, Kazunobu; Hiramatsu, Takaaki; Tokunaga, Etsuko; Dohi, Toshifumi; Kita, Yasuyuki; Shibata, Norio
Iodoarene-Catalyzed Fluorination and Aminofluorination by Ar-I/HF•Pyridine/mCPBA System.
Chem. Sci., 5, 2754-2760 (2014).
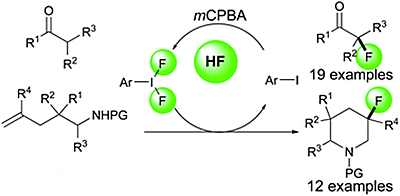
- Kita, Yasuyuki; Dohi, Toshifumi
Pioneering Metal-Free Oxidative Coupling Strategy of Aromatic Compounds Using Hypervalent Iodine Reagent.
Chem. Rec., 15, 886-906 (2015).
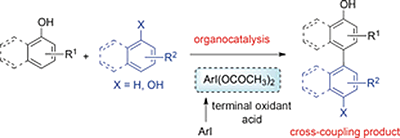
- Morimoto, Koji; Sakamoto, Kazuma; Ohshika, Takao; Dohi, Toshifumi; Kita, Yasuyuki
Organo-Iodine(III)-Catalyzed Oxidative Phenol–Arene and Phenol–Phenol Cross- Coupling Reaction.
Angew. Chem., Int. Ed., 55, 3652–3656 (2016).
The direct oxidative coupling reaction has been an attractive tool for environmentally benign chemistry. Reported herein is that the hypervalent iodine catalyzed oxidative metal-free cross-coupling reaction of phenols can be achieved using Oxone as a terminal oxidant in 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP). This method features a high efficiency and regioselectivity, as well as functional-group tolerance under very mild reaction conditions without using metal catalysts.
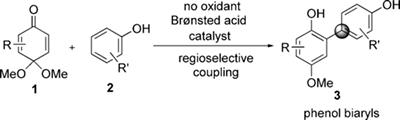
- Kamitanaka, Tohru; Morimoto, Koji; Tsuboshima, Kohei; Koseki, Daichi; Takamuro, Hitoho; Dohi, Toshifumi; Kita, Yasuyuki
Efficient Coupling Reaction of Quinone Monoacetal with Phenols Leading to Phenol Biaryls.
Angew. Chem., Int. Ed., 55, 15535-15538 (2016).
A simple and efficient synthesis of phenol biaryls by the cross-couplings of quinone monoacetals (QMAs) and phenols is reported. The Brønsted acid catalytic system in 1,1,1,3,3,3- hexafluoro -2-propanol was found to be particularly efficient for this transformation. This reaction can be extended to the synthesis of various phenol biaryls, including sterically hindered biaryls, with yields ranging from 58 to 90 % under mild reaction conditions and in a highly regiospecific manner.
Impact factors of the selected 40 papers (JCR 2021) are shown below
| Angew. Chem. Int. Ed. | 16.823 |
| J. Am. Chem. Soc. | 16.383 |
| Green Chem. | 11.034 |
| Chem. Sci. | 9.969 |
| Chem. Rec. | 6.935 |
| Chem. Commun. | 6.065 |
| Adv. Synth. Cat. | 5.981 |
| J.Org. Chem. | 4.198 |
| Tetrahedron | 2.388 |
Top 75 Papers by Total Citation Numbers
(Citation Numbers are shown in the parenthesis, 24 March, 2023)
- Dohi, Toshifumi; Kita, Yasuyuki.
Hypervalent Iodine Reagents as a New Entrance to Organocatalysts
Chem. Commun., 2009, 2073-2085.[666] - Dohi, Toshifumi; Maruyama, Akinobu; Takenaga, Naoko; Senami, Kento; Minamitsuji, Yutaka; Fujioka, Hiromichi; Caemmerer, Simon B; Kita, Yasuyuki
A Chiral Hypervalent Iodine(III) Reagent for Enantioselective Dearomatization of Phenols
Angew. Chem. Int. Ed., 2008, 47, 3787-3790.[388] - Kita, Yasuyuki; Tohma, Hirofumi; Hatanaka, Kenji; Takada, Takeshi; Fujita, Shigekazu; Mitoh, Shizue; Sakurai,Hiromu; Oka, Shigenori
Hypervalent Iodine-Induced Nucleophilic Substitution of para-Substituted Phenol Ethers Generation of Cation Radicals as Reactive Intermediates
J. Am. Chem. Soc., 1994, 116, 3684-3691.[381] - Tohma, Hirofumi; Kita, Yasuyuki
Hypervalent Iodine Reagents for the Oxidation of Alcohols and their Application to Complex Molecule Synthesis
Adv. Synth. Catal. , 2004, 346, 111-124.[340] - Tamura, Yasumitsu; Yakura, Takayuki; Haruta, Jun-ichi; Kita, Yasuyuki
Hypervalent Iodine Oxidation of p-Alkoxyphenols and Related Compounds: A General Route to p-Benzoquinone Monoacetals and Spiro Lactones
J. Org. Chem. , 1987, 52 , 3927-3930. [326] - Dohi, Toshifumi; Maruyama, Akinobu; Yoshimura, Misaki; Morimoto, Koji; Tohma, Hirofumi; Kita, Yasuyuki
Versatile Hypervalent-Iodine(III)-Catalyzed Oxidations
with m-Chloroperbenzoic Acid as a Cooxidant
Angew. Chem. Int. Ed. , 2005, 44, 6193-6196.[299] - Kita, Yasuyuki; Morimoto, Koji; Ito, Motoki; Ogawa, Chieko; Goto, Akihiro;
Dohi, Toshifumi
Metal-Free Oxidative Cross-Coupling of Unfunctionalized Aromatic Compounds
J. Am. Chem. Soc., 2009, 131, 1668-1669.[265] - Dohi, Toshifumi; Takenaga, Naoko; Nakae, Tomofumi; Toyoda, Yosuke; Yamasaki, Mikio; Shiro, Motoo; Fujioka, Hiromichi; Maruyama, Akinobu; Kita, Yasuyuki
Asymmetric Dearomatizing Spirolactonizaion of Naphthols Catalyzed by Spirobiindane-Based Chiral Hypervalent Iodine Species
J. Am. Chem. Soc. , 2013, 135, 4558-4566. [246] - Dohi, Toshifumi; Yamaoka, Nobutaka; Kita, Yasuyuki
Fluoroalcohols: Versatile Solvents in Hypervalent Iodine Chemistry and Syntheses of Diaryliodonium(III) Salts
Tetrahedron , 2010, 66, 5775-5785.[226] - Dohi, Toshifumi; Ito, Motoki; Morimoto, Koji; Iwata, Minako; Kita, Yasuyuki
Oxidative Cross-Coupling of Arenes Induced By Single-Electron Transfer Leading to Biaryls Using Organoiodine(III) Oxidants
Angew. Chem. Int. Ed. , 2008, 47, 1301-1304. [219] - Dohi, Toshifumi; Ito, Motoki; Yamaoka, Nobutaka; Morimoto, Koji; Fujioka, Hiromichi; Kita,Yasuyuki
Hypervalent Iodine(III): Selective and Efficient Single-Electron-Transfer (SET) Oxidizing Agent
Tetrahedron , 2009, 65, 10797-10815.[209] - Takada, Takeshi; Arisawa, Mitsuhiro; Gyoten, Michiyo; Hamada, Ryuji; Tohma, Hirofumi; Kita,Yasuyuki
Oxidative Biaryl Coupling Reaction of Phenol Ether Derivatives Using a Hypervalent Iodine(III) Reagent
J. Org. Chem. , 1998, 63, 7698-7706.[193] - Tohma, Hirofumi; Morioka, Hironori; Takizawa, Shinobu; Arisawa, Mitsuhiro; Kita, Yasuyuki
Efficient Oxidative Biaryl Coupling Reaction of Phenol Ether Derivatives Using Hypervalent Iodine(lll) Reagents
Tetrahedron , 2001, 57, 345-352.[190] - Kita, Yasuyuki; Takada, Takeshi; Tohma, Hirofumi
Hypervalent Iodine Reagents in Organic Synthesis: Nucleophilic Substitution of p-Substituted Phenol Ethers
Pure Appl. Chem. ,1996, 68, 627-630.[188] - Dohi, Toshifumi; Maruyama, Akinobu; Yoshimura, Misaki; Morimoto, Koji; Tohma, Hirofumi; Kita, Yasuyuki
First Hypervalent Iodine(III)-Catalyzed C-N Bond Forming Reaction: Catalytic Spirocyclization of Amides to N-Fused Spirolactams
Chem. Commun. , 2007, 12, 1224-1226.[182] - Kita, Yasuyuki; Tohma, Hirofumi; Kikuchi, Kazumi; Inagaki, Masanao; Yakura, Takayuki
Hypervalent Iodine Oxidation of N-Acyltyramines: Synthesis of Quinol Ethers, Spirohexadienones, and Hexahydroindol-6-ones
J. Org. Chem. , 1991, 56, 435-438. [176] - Kita, Yasuyuki; Tohma, Hirofumi; Inagaki, Masanao; Hatanaka, Kenji; Yakura, Takayuki Total Synthesis of Discorhabdin C: A General Aza Spiro Dienone Formation from O-Silylated Phenol Derivatives Using a Hypervalent Iodine Reagent
J. Am. Chem. Soc. , 1992, 114, 2175-2180.[175] - Tohma, Hirofumi; Takizawa, Shinobu; Maegawa, Tomohiro; Kita, Yasuyuki
Facile and Clean Oxidation of Alcohols in Water Using Hypervalent Iodine(III) Reagents
Angew. Chem. Int. Ed ., 2000, 39, 1306-1308. [172] - Dohi, Toshifumi; Ito, Motoki; Yamaoka, Nobutaka; Morimoto, Koji; Fujioka, Hiromichi; Kita,Yasuyuki
Unusual ipso Substitution of Diaryliodonium Bromides Initiated by a Single-Electron-Transfer Oxidizing Pprocess
Angew. Chem. Int. Ed. , 2010, 49, 3334-3337.[170] - Kita, Yasuyuki; Arisawa, Mitsuhiro; Gyoten, Michiyo; Nakajima, Makiko; Hamada, Ryuji; Tohma, Hirofumi; Takada, Takeshi
Oxidative Intramolecular Phenolic Coupling Reaction Induced by a Hypervalent Iodine(III) Reagent: Leading to Galanthamine-Type Amaryllidaceae Alkaloids
J. Org. Chem. , 1998, 63, 6625-6633.[160] - Kita, Yasuyuki; Takada, Takeshi; Gyoten, Michiyo; Tohma, Hirofumi; Zenk, Meinhart H.; Eichhorn, Joerg
An Oxidative Intramolecular Phenolic Coupling Reaction for the Synthesis of Amaryllidaceae Alkaloids Using a Hypervalent Iodine(III) Reagent
J. Org. Chem. , 1996, 64, 5857-5864.[143] - Kita, Yasuyuki; Tohma, Hirofumi; Inagaki, Masanao; Hatanaka, Kenji; Yakura, Takayuki
A Novel Oxidative Azidation of Aromatic Compounds with Hypervalent Iodine Reagent, Phenyliodine(III) Bis(Trifluoroacetate) (PIFA) and Trimethylsilyl Azide
Tetrahedron Lett. , 1991, 32, 4321-4324. [139] - Dohi, Toshifumi; Minamitsuji, Yutaka; Maruyama, Akinobu; Hirose, Satoshi; Kita, Yasuyuki
A New H2O2/Acid Anhydride System for the Iodoarene-Catalyzed C-C Bond-Forming Reactions of Phenols
Org. Lett. , 2008, 10, 3559-3562. [137] - Suzuki, Satoru; Kamo, Tomohiro; Fukushi, Kazunobu; Hiramatsu, Takaaki; Tokunaga, Etsuko;Dohi, Toshifumi, Kita, Yasuyuki; Shibata, Norio
Iodoarene-Catalyzed Fluorination and Aminofluorination by an Ar-I/HF.Pyridine/mCPBA System
Chem. Sci., 2014, 5, 2754-2760. [133] - Tohma, Hirofumi; Takizawa, Shinobu; Watanabe, Hiroaki; Fukuoka, Yuko;
Maegawa, Tomohiro; Kita, Yasuyuki
Hypervalent Iodine(V)-Induced Asymmetric Oxidation of Sulfides to Sulfoxides Mediated by Reversed Micelles: Novel Nonmetallic Catalytic System
J. Org. Chem. , 1999, 64, 3519-3523. [131] - Dohi, Toshifumi; Morimoto, Koji; Maruyama, Akinobu; Kita, Yasuyuki
Direct Synthesis of Bipyrroles Using Phenyliodine Bis(trifluoroacetate) with Bromotrimethylsilane
Org. Lett., 2006, 8, 2007-2010. [127] - Dohi, Toshifumi; Takenaga, Naoko; Goto, Akihiro; Fujioka, Hiromichi; Kita, Yasuyuki
Clean and Efficient Benzylic C-H Oxidation in Water Using a Hypervalent Iodine Reagent: Activation of Polymeric Iodosobenzene with KBr in the Presence of Montmorillonite-K10
J. Org. Chem. , 2008, 73, 7365-7368.[126] - Ito, Motoki; Kubo, Hiroko; Itani, Itsuki; Morimoto, Koji; Dohi, Toshifumi; Kita, Yasuyuki
Organocatalytic C-H/C-H' Cross-Biaryl Coupling: C-Selective Arylation of Sulfonanilides with Aromatic Hydrocarbons
J. Am. Chem. Soc. , 2013, 38, 14078-14081. [124] - Kita, Yasuyuki; Higuchi, Kazuhiro; Yoshida, Yutaka; Iio, Kiyosei; Kitagaki, Shinji; Ueda, Koichiro; Akai, Shuji; Fujioka, Hiromichi
Enantioselective Total Synthesis of a Potent Antitumor Antibiotic, Fredericamycin A
J. Am. Chem. Soc. , 2001, 123, 3214-3222.[119] - Fujioka, Hiromichi; Murai, Kenichi; Ohba, Yusuke; Hiramatsu, Atsushi; Kita, Yasuyuki
A Mild and Efficient One-Pot Synthesis of 2-Dihydroimidazoles from Aldehydes
Tetrahedron Lett. , 2005, 46, 2197-2199. [117] - Kita, Yasuyuki; Gyoten, Michiyo; Ohtsubo,Makoto; Tohma, Hirofumi; Takada, Takeshi
Non-Phenolic Oxidative Coupling of Phenol Ether Derivatives Using Phenyliodine(III) Bis(trifluoroacetate)
Chem. Commun. , 1996, 1481-1482.[116] - Kita, Yasuyuki; Takada, Takeshi; Mihara, Sachiko; Whelan, Brendan A.; Tohma, Hirofumi
Novel and Direct Nucleophilic Sulfenylation and Thiocyanation of Phenol Ethers Using a Hypervalent Iodine(III) Reagent
J. Org. Chem. , 1995, 60, 7144-7148.[115] - Kita, Yasuyuki; Akai, Shuji; Ajimura, Naomi; Yoshigi, Mayumi; Tsugoshi, Teruhisa; Yasuda, Hitoshi; Tamura, Yasumitsu
Facile and Efficient Syntheses of Carboxylic Anhydrides and Amides Using (Trimethylsilyl)ethoxyacetylene
J. Org. Chem. , 1986, 51, 4150-4158.[115] - Akai, Shuji; Tanimoto, Kouichi; Kanao, Yukiko; Egi, Masahiro; Yamamoto, Tomoko; Kita,Yasuyuki
A Dynamic Kinetic Resolution of Allyl Alcohols by the Combined Use of Lipases and [VO(OSiPh3)3]
Angew. Chem. Int. Ed. , 2006, 45, 2592-2595. [114] - Akai, Shuji; Kita, Yasuyuki
Recent Advances in Pummerer Reactions
Top. Curr. Chem. , 2007, 274(Characterization I), 35-76.[113] - Matsugi, Masato; Murata, Kenji; Gotanda, Kentoku; Nambu, Hisanori; Anilkumar, Gopinathan; Matsumoto, Keita; Kita,
Yasuyuki
Facile and Efficient Sulfenylation Method Using Quinone Mono-O,S-Acetals Under Mild Conditions
J. Org. Chem. , 2001, 66, 2434-2441.[113] - Kita, Yasuyuki; Segawa, Jun; Haruta, Jun-ichi; Yasuda, Hitoshi; Tamura, Yasumitsu
Ketene Silyl Acetal Chemistry; Simple Synthesis of Methyl Jasmonate and Related Compounds by Utilising Ketene Methyl Dimethyl-tert-Butylsilyl Acetal
J. Chem. Soc. Perkin Trans. , 1982, 51, 1099-1104.[108] - Kita, Yasuyuki; Dohi, Toshifumi
Pioneering Metal-Free Oxidative Coupling Strategy of Aromatic Compounds
Using Hypervalent Iodine Reagents
Chem. Rec. , 2015, 15, 886-906.[107] - Dohi, Toshifumi; Takenaga, Naoko; Goto, Akihiro; Maruyama, Akinobu; Kita, Yasuyuki
Direct Lactone Formation by Using Hypervalent Iodine(III) Reagents with KBr via Selective C-H Abstraction Protocol
Org. Lett. , 2007, 9, 3129-3132.[105] - Dohi, Toshifumi; Morimoto, Koji; Takenaga, Naoko; Goto, Akihiro; Maruyama, Akinobu; Kiyono, Yorito; Tohma, Hirofumi; Kita, Yasuyuki
Direct Cyanation of Heteroaromatic Compounds Mediated by Hypervalent Iodine(III) Reagents: In situ Generation of PhI(III)-CN Species and their Cyano Transfer
J. Org. Chem., 2007, 72, 109-116.[103] - Dohi, Toshifumi; Ito, Motoki; Morimoto, Koji; Minamitsuji Yutaka; Takenaga, Naoko; Kita, Yasuyuki
Versatile Direct Dehydrative Approach for Diaryliodonium(III) Salts in Fluoroalcohol Media
Chem. Commun., 2007, 4152-4154.[99] - Fujioka, Hiromichi; Okitsu, Takashi; Sawama, Yoshinari; Murata, Nobutaka; Li, Ruichuan; Kita, Yasuyuki
Reaction of the Acetals with TESOTf-Base Combination; Speculation of the Intermediates and Efficient Mixed Acetal Formation
J. Am. Chem. Soc. , 2006, 128, 5930-5938.[99] - Tohma, Hirofumi; Maegawa, Tomohiro; Takizawa, Shinobu; Kita, Yasuyuki
Facile and Clean Oxidation of Alcohols In Water Using Hypervalent Iodine(III) Reagents
Adv. Synth. Catal. , 2002, 344, 328-337. [99] - Kita, Yasuyuki; Yakura, Takayuki; Tohma, Hirofumi; Kikuchi, Kazumi; Tamura, Yasumitsu
A Synthetic Approach to Discorhabdin Alkaloids: Hypervalent Iodine Oxidation of p-Substituted Phenol Derivatives to Azacarbocyclic Spirodienones
Tetrahedron Lett. , 1989, 30, 1119-1120.[98] - Dohi, Toshifumi; Morimoto, Koji; Kiyono, Yorito; Tohma, Hirofumi; Kita, Yasuyuki
Novel and Direct Oxidative Cyanation Reactions of Heteroaromatic Compounds Mediated by a Hypervalent Iodine(III) Reagent
Org. Lett. , 2005, 7, 537-540. [97] - Tohma, Hirofumi; Morioka, Hironori; Harayama, Yu; Hashizume, Miki; Kita, Yasuyuki
Novel and Efficient Synthesis of p-Quinones in Water via Oxidative Demethylation of Phenol Ethers Using Hypervalent Iodine(lll) Reagents
Tetrahedron Lett. , 2001, 42, 6899-6902.[97] - Kita, Yasuyuki; Nambu, Hisanori; Ramesh, Namakkal G.; Anilkumar, Gopinathan; Matsugi, Masato
A Novel and Efficient Methodology for the C-C Bond Forming Radical Cyclization of Hydrophobic Substrates in Water
Org. Lett. , 2001, 3, 1157-1160.[97] - Hamamoto, Hiromi; Anilkumar, Gopinathan; Tohma, Hirofumi; Kita, Yasuyuki
A Novel and Useful Oxidative Intramolecular Coupling Reaction of Phenol Ether Derivatives on Treatment with a Combination of Hypervalent Iodine(III) Reagent and Heteropoly Acid
Chem. Eur. J. , 2002, 8, 5377-5383.[94] - Morimoto, Koji; Sakamoto, Kazuma; Ohnishi, Yusuke; Miyamoto, Takeshi; Ito, Motoki; Dohi, Toshifumi; Kita,Yasuyuki
Metal-Free Oxidative para Cross-Coupling of Phenols
Chem. Eur. J. , 2013, 19, 8726-8731.[93] - Tamura, Yasumitsu; Yakura, Takayuki; Tohma, Hirofumi; Kikuchi, Koichi; Kita, Yasuyuki
Hypervalent Iodine Oxidation of p-Alkoxy-Phenol and Related Phenol-A Facile and Efficient Synthesisof p-Quinones
Synthesis , 1989, 126-127. [92] - Tamura, Yasumitsu; Sasho, Manabu; Nakagawa, Kiyomi; Tsugoshi, Teruhisa; Kita, Yasuyuki
Strong Base Induced Cycloaddition of Homophthalic Anhydrides Leading to p-Hydroxy Polycyclic Compounds
J. Org. Chem. , 1984, 60, 473-478.[89] - Tohma, Hirofumi; Maruyama, Akinobu; Maeda, Akihisa; Maegawa, Tomohiro; Dohi, Toshifumi; Shiro, Motoo; Morita, Tetsuichiro; Kita, Yasuyuki
Preparation and Reactivity of 1,3,5,7-Tetrakis[4-(Diacetoxyiodo)Phenyl]Adamantane, a Recyclable Hypervalent Iodine(III) Reagent
Angew. Chem. Int. Ed. , 2004, 43, 3595-3598. [86] - Morimoto, Koji; Sakamoto, Kazuma; Ohshika, Takao; Dohi, Toshifumi; Kita, Yasuyuki
Organo-Iodine(III)-Catalyzed Oxidative Phenol-Arene and Phenol-Phenol Cross-Coupling Reaction
Angew. Chem. Int. Ed. , 2016, 55, 3652-3656.[85] - Arisawa, Mitsuhiro; Utsumi, Saori; Nakajima, Makiko; Ramesh, Namakkal G.; Tohma, Hirofumi; Kita, Yasuyuki
An Unexpected Intermolecular Chiral Biaryl Coupling Reaction Induced by a Hypervalent Iodine(III) Reagent: Synthesis of Potential Precursor for Ellagitannin
Chem. Commun. , 1999, 469-470. [84] - Eichhorn, Jorg; Takada, Takeshi; Kita, Yasuyuki; Zenk, Meinhart, H.
Biosynthesis of the Amaryllidaceae Alkaloid Galanthamine
Phytochemistry , 1998, 49, 1037-1047. [82] - Dohi, Toshifumi; Nakae, Tomofumi; Ishikado, Yohei; Kato, Daishi; Kita, Yasuyuki
New Synthesis of Spirocycles by Utilizing in situ Forming Hypervalent Iodine Species
Org. Biomol. Chem. , 2011, 9, 6899-6902. [81] - Morimoto, Koji; Yamaoka, Nobutaka; Ogawa, Chieko; Nakae, Tomofumi; Fujioka, Hiromichi; Dohi, Toshifumi; Kita,Yasuyuki
Metal-Free Regioselective Oxidative Biaryl Coupling Leading to Head-to-Tail Bithiophenes: Reactivity Switching, a Concept Based on the Iodonium(III) Intermediate
Org. Lett. , 2010, 12, 3804-3807.[80] - Akai, Shuji; Hanada, Ryosuke; Fujiwara, Noboru; Kita, Yasuyuki; Egi, Masahiro
One-Pot Synthesis of Optically Active Allyl Esters via Lipase-Vanadium Combo Catalysis
Org. Lett., 2010, 12, 4900-4903. [79] - Tohma, Hirofumi; Takizawa, Shinobu; Watanabe, Hiroaki; Kita, Yasuyuki
Hypervalent Iodine(III) Oxidation Catalyzed by Quaternary Ammonium Salt in Micellar Systems
Tetrahedron Lett. , 1998, 39, 4547-4550. [79] - Dohi, Toshifumi; Ito, Motoki; Itani, Itsuki; Yamaoka, Nobutaka; Morimoto, Koji; Fujioka, Hiromichi; Kita, Yasuyuki
Metal-free C-H Cross-Coupling Toward Oxygenated Naphthalene-Benzene Linked Biaryls
Org. Lett., 2011, 13, 6208-6211. [78] - Tohma, Hirofumi; Harayama, Yu; Hashizume, Miki; Iwata, Minako; Kiyono, Yorito; Egi, Masahiro; Kita, Yasuyuki
The First Total Synthesis of Discorhabdin A
J. Am. Chem. Soc. , 2003, 125, 11235-11240. [77] - Akai, Shuji; Ikawa, Takashi; Takayanagi, Sho-ichi; Morikawa, Yuki; Mohri, Shinya; Tsubakiyama, Masaya; Egi, Masahiro; Wada, Yasufumi; Kita, Yasuyuki
Synthesis of Biaryl Compounds Through Three-Component Assembly: Ambidentate Effect of the Tertbutyldimethylsilyl Group for Regioselective Diels-Alder and Hiyama Coupling Reactions
Angew. Chem. Int. Ed. , 2008, 47, 7673-7676. [76] - Tamura, Yasumitsu; Ishibashi, Hiroyuki; Hirai, Michio; Kita, Yasuyuki; Ikeda, Masazumi
Photochemical Syntheses of 2-Azabicyclo and 2-Oxabicyclo [2.1.1]hexane Ring-Systems
J. Org. Chem. , 1975, 40, 2702-2710.[76] - Dohi, Toshifumi; Kato, Daishi; Hyodo, Ryo; Yamashita, Daisuke; Shiro, Motoo; Kita, Yasuyuki
Discovery of Stabilized Bisiodonium Salts as Intermediates in the Carbon-Carbon Bond Formation of Alkynes
Angew. Chem. Int. Ed. , 2011, 50, 3784-3787.[75] - Arisawa, Mitsuhiro; Ramesh, Namakkal G.; Nakajima, Makiko; Tohma, Hirofumi; Kita, Yasuyuki
Hypervalent Iodine(III)-Induced Intramolecular Cyclization of α-(Aryl)alkyl-β-dicarbonyl Compounds: A Convenient Synthesis of Benzannulated and Spirobenzannulated Compounds
J. Org. Chem., 2001, 66, 59-65. [75] - Kita, Yasuyuki; Egi, Masahiro; Takada, Takeshi; Tohma, Hirofumi
Development of Novel Reactions Using Hypervalent Iodine(III) Reagents. Total Synthesis of Sulfur-Containing Pyrroloiminoquinone Marine Product, (±)-Makaluvamine F
Synthesis, 1999, 885-897. [75] - Kita, Yasuyuki; Maeda, Hiroshi; Omori, Kana; Okuno, Takayuki; Tamura, Yasumitsu
Novel Efficient Synthesis of 1-Ethoxyvinyl Esters Using Ruthenium Catalysts and Their Use In Acylation of Amines and Alcohols: Synthesis of Hydrophilic 3'-N-Acylated Oxaunomycin Derivatives
J. Chem. Soc. Perkin Trans. , 1993, 51, 2999-3005.[75] - Tamura, Yasumitsu; Yakura, Takayuki; Haruta, Jun-ichi; Kita, Yasuyuki
An Efficient Conversion of Keto Groups Into Dihydroxyacetone Groups: Oxidation of Ethynylcarbinol Intermediates by Using Hypervalent Iodine Reagent
Tetrahedron Lett. , 1985, 26, 3837-3840.[75] - Kita, Yasuyuki; Haruta, Jun-ichi; Segawa, Jun; Tamura, Yasumitsu
Ketene Methyl Trialkylsilyl Acetals as Effective Silylating Agents for Alcohols. Carboxylic Acids, Mercaptans, and Amides
Tetrahedron Lett. , 1979, 20, 4311-4314.[75] - Dohi, Toshifumi; Takenaga, Naoko; Fukushima, Kei-ichiro; Uchiyama, Teruyoshi; Kato, Daishi; Shiro, Motoo; Fujioka, Hiromichi; Kita, Yasuyuki
Designer μ-oxo-bridged Hypervalent Iodine(III) Organocatalysts for Greener Oxidations
Chem. Commun. , 2010, 46, 7697-7699.[74] - Tohma, Hirofumi; Kita, Yasuyuki
Synthetic Applications (Total Synthesis and Natural Product Synthesis): Hypervalent Iodine Chemistry
Top. Curr. Chem., 2003, 224, 209-248.[73] - Akai, Shuji; Tanimoto, Kouichi; Kita, Yasuyuki
Lipase-Catalyzed Domino Dynamic Kinetic Resolution of Racemic 3-Vinylcyclohex-2-en-1-ols/Intramolecular Diels-Alder Reaction: One-Pot Synthesis of Optically Active Polysubstituted Decalins
Angew. Chem. Int. Ed. , 2004, 43, 1407-1410.[71] - Kita, Yasuyuki; Futamura, Junko; Ohba, Yusuke; Sawama, Yoshinari; Ganesh, Jnaneshwara K.; Fujioka, Hiromichi
The Rearrangement of 2,3-Epoxysulfonates and Its Application to
Natural Products Syntheses: Formal Synthesis of (-)-Aphanorphine and Total Syntheses of (-)-α-Herbertenol and (-)-Herbertenediol
J. Org. Chem. , 2003, 68, 5917-5924. [71] - Tamura, Yasumitsu; Kawasaki, Tomomi; Adachi, Masahiro; Tanio, Masami; Kita, Yasuyuki
Thiocyanation and Cyanation Using a New Combined Reagent of Triphenylphosphine and Thiocyanogen
Tetrahedron Lett. , 1977, 50, 4417-4420. [71] - Dohi, Toshifumi; Morimoto, Koji; Kiyono, Yorito; Maruyama, Akinobu; Tohma, Hirofumi; Kita, Yasuyuki
The Synthesis of Head-to-tail (H-T) Dimers of 3-Substituted Thiophenes by the Hypervalent Iodine(III)-Induced Oxidative Biaryl Coupling Reaction
Chem. Commun. , 2005, 23, 2930-2932. [70]
Published more than 575 original papers and the total citation numbers is 20,776 (h-index 71)
Impact factors of the top 75 papers (JCR 2021) are shown below
| Angew. Chem. Int. Ed. | 16.823 | J. Am. Chem. Soc. | 16.383 |
| Green Chem. | 11.034 | Chem. Sci. | 9.969 |
| J. Med. Chem. | 8.039 | Chem. Rec. | 6.935 |
| Chem. Commun. | 6.065 | Org. Lett. | 6.072 |
| Adv.Synth. Catal. | 5.981 | Chem. Eur. J. | 5.02 |
| Chem. Asian. J. | 4.839 | J. Org. Chem. | 4.198 |
| Org. Biomol. Chem. | 3.89 | Eur. J. Org. Chem. | 3.261 |
| Synthesis | 3.019 | Tetrahedron | 2.388 |
| Pure Appl. Chem. | 2.32 | Synlett | 2.206 |
| Tetrahedron Lett. | 2.032 |