Research
(1) Supported Metal Catalyst
Outline
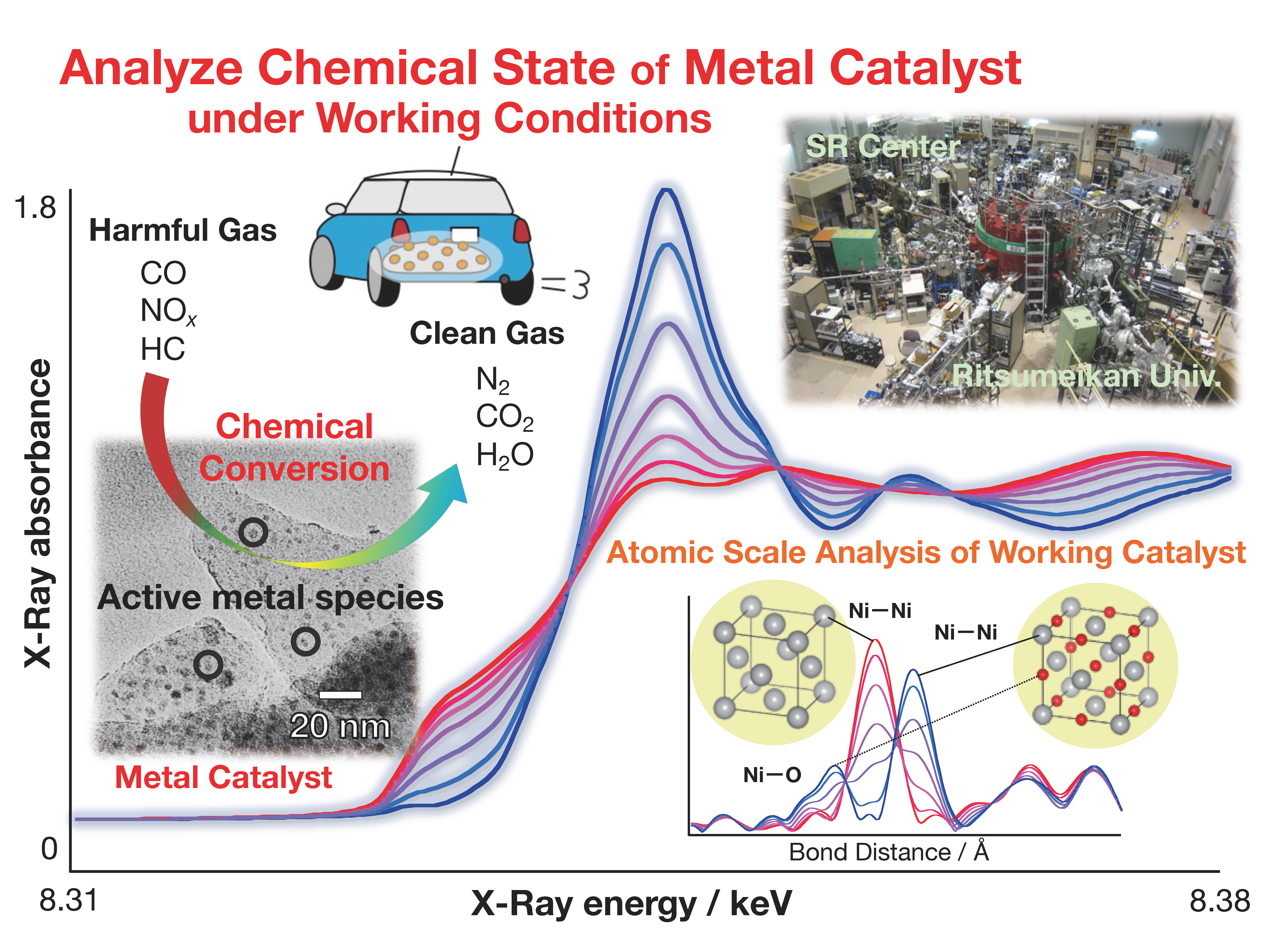
Supported metal catalysts play an important role in many situations, such as the efficient production of various materials and the purification of toxic exhaust gasses from factories and automobiles. The development of higher performance catalysts is essential for the establishment of a sustainable society in the future. Instead of the traditional trial-and-error development, we aim to elucidate the reaction mechanism of the current catalysis system at the atomic scale under working conditions. Through understanding the factors that limit performance, we will present guiding principles for more efficient performance, reduction of side reactions, cost saving, and long life.
It is important to know the chemical state of active metal species in the environment in which the catalyst actually reacts (high temperature or reaction gas atmosphere). For example, when the reaction proceeds by dissociation of a chemical bond with metallic particles, the activity disappears if the particle surface becomes the oxide. By analyzing the chemical state of metallic nanoparticles under reaction conditions, it is possible to find out the factors that limit the performance. The improvement of catalytic activity is achieved by preventing the factors. For such research purposes, the XAFS analysis using synchrotron radiation facilities is very effective. We aim to create future catalyst materials by applying the XAFS analysis technique that we have developed so far.
Research Example
Simultaneous Detoxification of NOX and CO
S. Yamashita, Y. Yamamoto, H. Kawabata, Y. Niwa, M. Katayama, and Y. Inada, Catal. Today, 303(1), 33-39 (2018).
Exhaust gas generated from fossil fuels includes hazardous components, such as NOx and CO. For example, a three-way catalyst is widely used as the catalyst that detoxifies the automobile exhaust gas, but it contains expensive rare metals. This is an example of research to reduce the cost.
The next figure shows the result of applying the XAFS technique, which is measured under the reaction environment, to a Ni catalyst supported on silica. The left figure shows that metallic Ni is oxidized to NiO from 300 °C to 600 °C (at this time, NO is reduced to N2) under a NO atmosphere. On the other hand, it is shown in the right figure that NiO is reduced to metallic Ni at about 550 °C (at this time, CO is oxidized to CO2) under a CO atmosphere.
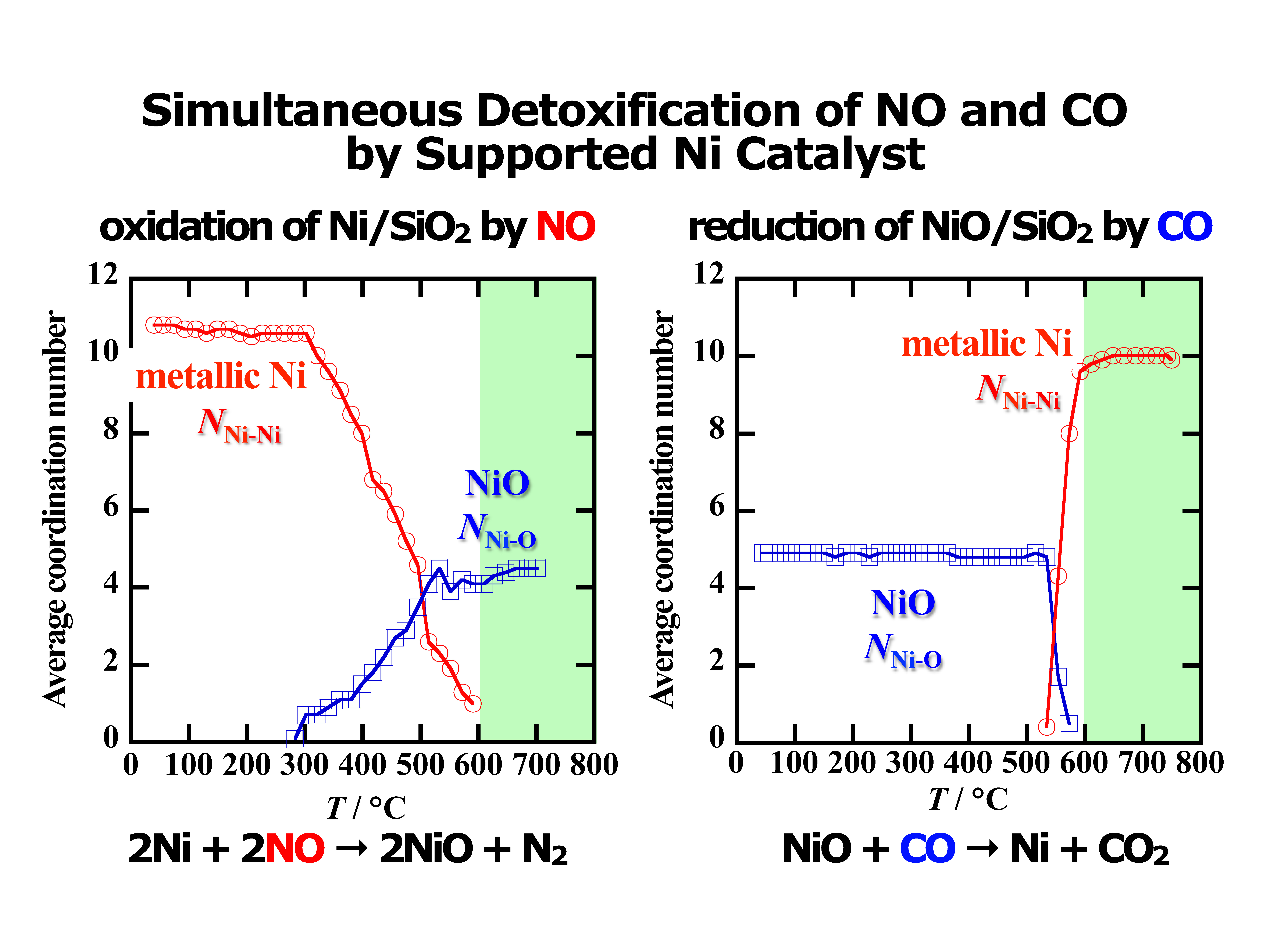
From this result, if NO and CO coexist at above 600 °C, the oxidation of the supported Ni species by NO and the reduction by CO proceed at the same time, resulting in the reduction of NO to N2 and the oxidation of CO to CO2. You can see that the simultaneous detoxification of NO and CO will be achieved using the supported Ni catalyst. Although the requirement of high temperature is still a problem, it is expected as a new rare-metal-free exhaust gas purification catalyst.
(2) Rechargeable Battery
Outline
In order to solve the energy problems faced by modern society, research on improving the performance of secondary batteries is being widely conducted. The development of electrode materials with high capacity, long life, and high stability is being carried out. Instead of the traditional trial-and-error development of the electrode materials, we elucidate the reaction mechanism of the current battery systems, especially from the perspective of the spatial scale, and clarify the factors that limit the battery performance. We aim to create higher-performance secondary battery materials that overcome the restrictions of current battery systems.
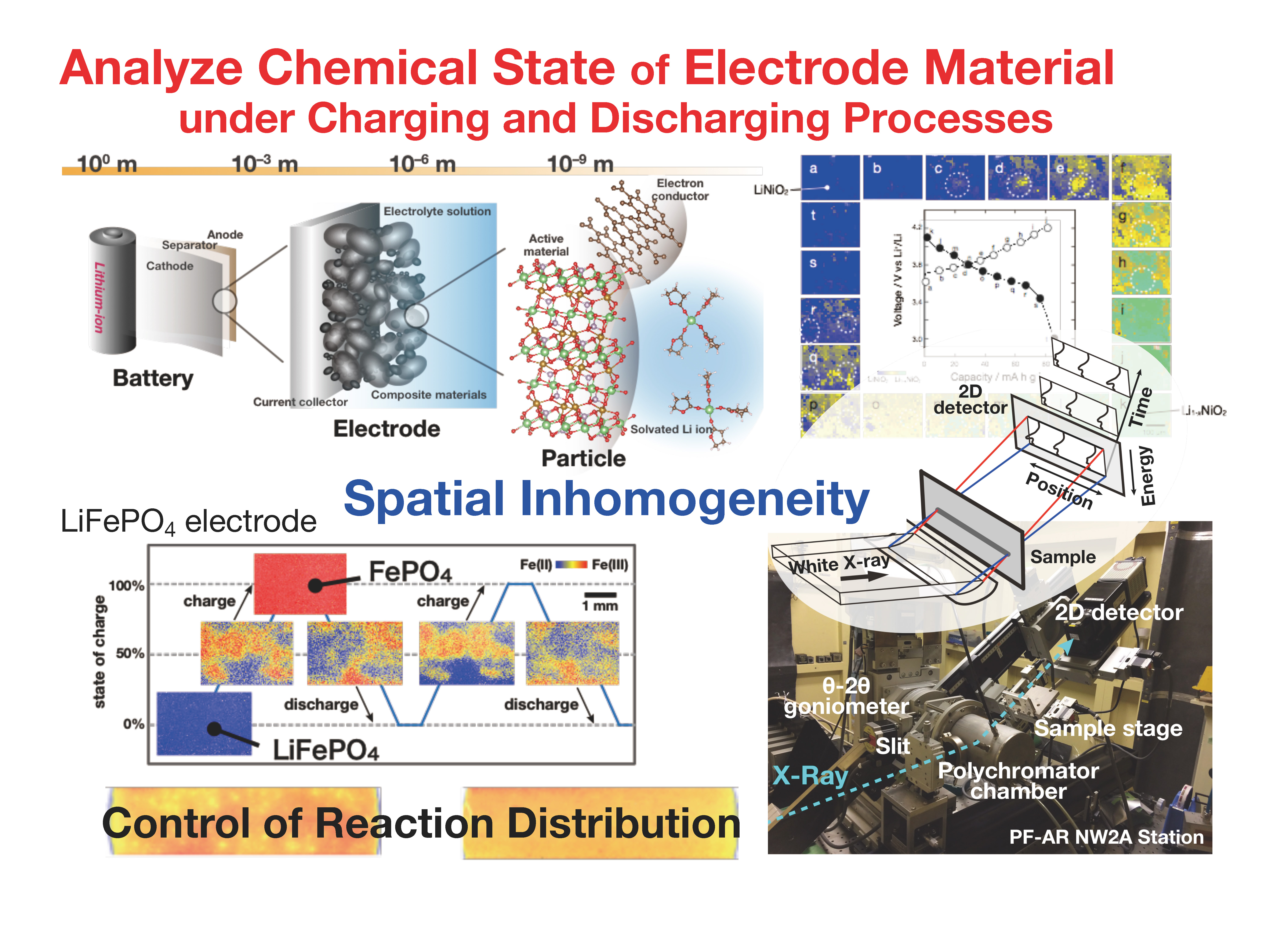
In this research as well, it is important to know the chemical state of the electrode active material during the charge and discharge processes. For example, in the positive electrode of a secondary battery, electrons are emitted at the charge process (the active material is oxidized), and electrons are incorporated at the discharge process (the active material is reduced). In addition, because the electrodes of the secondary battery are in the form of a sheet, the redox reaction is proceeding everywhere in the two-dimensional sheet. We have developed the XAFS method, which analyzes the oxidation state of the active material for each location on the two-dimensional sheet in order to visualize how the charge/discharge reaction of the secondary battery is proceeding. Then, we aim to clarify the problems hidden in them and will present guidelines for solving the problems.
Research Example
Spatial Inhomogeneity of Lithium Iron Phosphate Cathode
M. Katayama, K. Sumiwaka, R. Miyahara, H. Yamashige, H. Arai, Y. Uchimoto, T. Ohta, Y. Inada, and Z. Ogumi, J. Power Sources, 269, 994-999 (2014).
Lithium iron phosphate (LiFePO4, LFP) is one of the positive electrode active materials of lithium-ion secondary batteries. It is stable even if the Li ions are completely extracted (all Fe is oxidized) by the charge process, and in addition it is possible that the Li ions are reversibly inserted (all Fe is reduced) by the discharge process. However, its low electrical conductivity is a serious issue as the active materials of battery. As shown in the next figure, the existence of areas is clearly observed in the sheet-shaped positive electrode at where the charge/discharge reaction is easy/difficult to proceed.
We have originally developed the XAFS technique for spatially decomposing a two-dimensional sheet, and successfully visualized the charge/discharge reactions that progress heterogeneously. It was found that the spatial non-uniformity of the electrical conductivity of the composite material (mixture of LFP, carbon, and binder) applied to the current collector foil was the origin to generate the inhomogeneous reaction. Based on the results, we succeeded in improving the non-uniformity by the conductive coating on the surface of the current collector foil and greatly improving the large current discharge performance by modifying the carbon nanotubes on the LFP particles.

(3) Development of Advanced XAFS Technique
Outline
The XAFS technique allows you to select an element and analyze the electronic state and local structure of the target element without any restrictions of the sample phase (solid, liquid, gas) and conditions (high/low temperature, gas atmosphere). For materials such as metal catalysts and secondary battery materials in which metal elements exhibit their functions in the solid state, the in-situ XAFS analysis provides extremely useful information. We have been researching the XAFS technology for about 30 years, and we have developed wide variety of new XAFS measurement techniques. For example, the stopped-flow time-resolved DXAFS method, the two-crystal DXAFS method, the pump & probe DXAFS method, the two-dimensional imaging XAFS method, the vertically dispersive one-dimensional DXAFS method, and the two-element DXAFS method (see Publication).
New development of such experimental methods requires specialized knowledge and technology, but if we can create the world's first experimental equipment that has never existed, we will be able to obtain the world's first experimental data that has never observed before. It makes it possible to elucidate the reaction mechanism that was unknown so far, and it gives important information for the creation of new functional materials.

